University of Arizona scientists help uncover a rare brain disorder
When Dr. Martha Bhattacharya first studied a little-known gene called TMEM184B, she wasn’t looking for a childhood disorder. At the time, she was a postdoctoral researcher investigating how neurons respond to injury, work that she hoped might one day help people with neurodegenerative diseases like ALS.
“I didn’t know it was a disease gene,” Bhattacharya said. “I just knew it was really conserved – the same in fruit flies, mice, and humans – which usually means it’s doing something important.”
That curiosity set off a years-long journey that would lead her lab at the University of Arizona to help identify a rare neurodevelopmental syndrome, one that connects how the brain develops with how its cells manage energy.
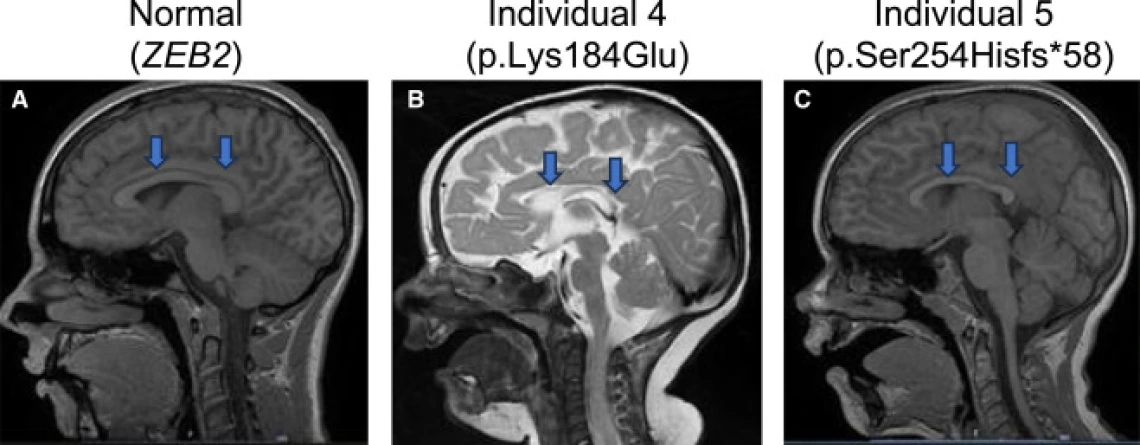
The team’s findings were recently published in The American Journal of Human Genetics in collaboration with researchers from the U.S., Europe, and beyond. The paper identifies mutations in TMEM184B as the cause of a new neurodevelopmental condition marked by developmental delay, seizures, and changes in brain structure.
A gene hidden in plain sight

Dr. Martha Bhattacharya
The path from fruit fly gene to a human diagnosis was not a straight line. After years studying TMEM184B in animals, Bhattacharya attended a conference where another scientist suggested she post her research on a platform that connects geneticists with clinicians studying rare diseases.
“I put my information on there,” she said.”And I started getting connections from genetic counselors and pediatricians who had patients with unknown disorders and mutations in this gene.”
As she began hearing from doctors around the world a pattern started to emerge. Each patient’s genome carried a different mutation in TMEM184B, yet their symptoms looked very similar.
Proving the link
Connecting the dots between genetics and disease is never simple.
“One hurdle is proving that a mutation is actually causative,” Bhattacharya said. “Human genomes have a lot of variation.”
Her lab, along with collaborators at Lurie Children's Hospital at Northwestern University, turned to model organisms for answers. By removing or replacing the gene in fruit flies and zebrafish, they showed that disruption of TMEM184B causes the same types of neural defects seen in patients – smaller brains, altered neuron growth, and seizure-like activity.

Zachary Yahiku
The next step was to understand why this gene’s loss had such widespread effects. That work fell partly to Zachary Yahiku, a graduate student in Bhattacharya’s lab.
Using fluorescent microscopy and flow cytometry, Yahiku studied how TMEM184B variants changed cellular health and stress signaling. He tagged a key stress-responsive protein with a green fluorescent marker to visualize how cells respond to metabolic stress.
“It was interesting how the data we were generating agreed with previous lab data on metabolism,” Yahiku said. “It felt like another piece of the puzzle falling into place.”
A surprising turn
The biggest surprise, Bhattacharya said, was realizing that TMEM184B’s main role wasn’t in degeneration, but in development.
“We thought it was going to be involved in a disease like ALS,” she said. “Instead, we had pediatric kids with epilepsy and brain structure disruption. It really made me take a 180 degree turn.”
Her team’s analysis found TMEM184B mutations after how cells handle amino acids and other metabolic pathways that fuel brain growth. She said that connection between metabolism and neurodevelopment could change how scientists think about early brain disorders.
Although the syndrome is extremely rare with only six patients being documented so far, Bhattacharya believes understanding it could help families find answers and open new therapeutic doors.
“Four of the six kids have seizures,” she said. “If we can figure out how this protein influences how neurons become overexcitable, maybe we can even find dietary or metabolic ways to help before we know how to drug this protein.”
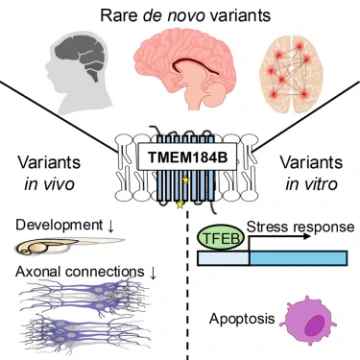
Her lab is now working with patient-derived stem cells, including one line created from a child in the study. The cells can be turned into neurons in the lab, allowing researchers to observe how different mutations affect brain activity and whether changing nutrients in the cells' environment can calm overactive neurons.
Collaboration and curiosity
For both Bhattacharya and Yahiku, the project was defined by collaboration. The global team met monthly, sharing data and troubleshooting across time zones.
“It tells you that science isn’t just sitting in your lab hoping for results,” Bhattacharya said. “It’s people sharing ideas and that makes science better.”
For Yahiku, who joined the lab as a first-year graduate student,the project became an education in both technique and perseverance.
“It was my rotation project that turned into my dissertation,” he said. “Seeing it go from an idea to a published paper taught me how to think critically and see the bigger picture.”
What began as a basic question about nerve injury in fruit flies has now touched families across the world. For Bhattacharya, it’s a reminder of why she took a chance on an uncertain gene few had studied before.
“As a scientist, sometimes you just follow your curiosity and hope it leads somewhere," she said. “This time it did and it led us to something that really matters.”
To learn more about the study, click here.

